“First we had to very quickly develop a way to process at DWPF without the salt waste, and then we had to incorporate the revised salt processing methods and streams into DWPF.” – Michael Stone, SRNL Engineer

The 2F Evaporator at the Savannah River Site. photo: Savannah River Site Photography
The Department of Energy (DOE) Office of Environmental Management is responsible for roughly 90 million gallons of radioactive liquid waste at Idaho National Laboratory (INL), the Office of River Protection at Hanford, Wash., and the Savannah River Site (SRS). About 56 million gallons are stored at Hanford, Wash., 900,000 gallons are at INL and roughly 36 million are stored at SRS. The Oak Ridge Site in Tennessee, also has approximately 400,000 gallons of waste from various operations, however that waste is not from reprocessing spent nuclear fuel.
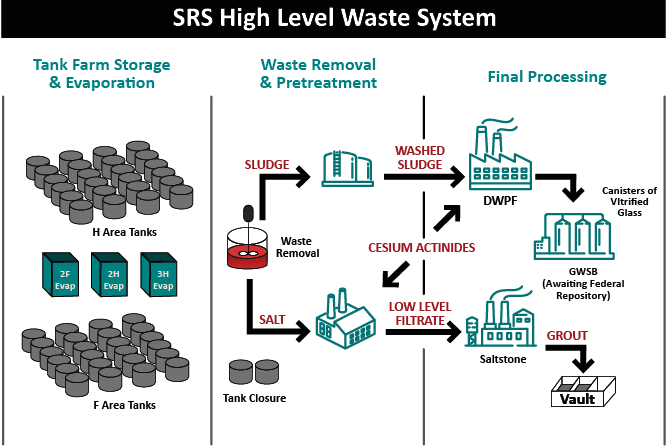
Millions of gallons of liquid waste are part of the legacy of America’s race to win the Cold War. The production of plutonium for nuclear weapons to defend our nation that began nearly 70 years ago resulted in high level radioactive waste, which is a mix of liquid, sediment, salts and sludge. A treatment path for these wastes was not available, so the waste was simply stored in large underground storage tanks. The tanks were arranged in groups which soon became known as tank “farms.” These tanks have outlived their design lives, posing a threat to the environment. Some of the tanks have known leaks.
“The radionuclides in the tank waste are essentially all the daughter products from the fission process that weren’t considered useful for other reasons,” said Michael Stone, an engineer and program lead for the Savannah River National Laboratory (SRNL) DOE HQ Technology Development, Lab Policy Office, Hanford WRPS Integrated Flowsheet and Hanford ORP Real-Time In-Line Monitoring. “For example, the plutonium, uranium, neptunium, americium and curium were extracted, but the processes were not 100% efficient and some of these materials were later determined to be excess and got put back into the tank farm. But the most radioactivity in the waste comes from the cesium and strontium,” Stone said.
But radionuclides aren’t the only ingredients in the waste that have to be handled.
“When you go through reprocessing, you end up adding a lot of chemicals to first dissolve the fuel rod material. That’s where the nitric acid is used and that’s where a lot of the nitrate comes from that’s present in the waste,” said Stone.
Stone also noted that mercury was added to some fuel rods for the dissolution process. “Then there’s some scavenging that’s done, to get rid of what they called the ‘do-bads’ – that’s the official term – that impacted the separations process and that is where some of the magnesium came from and some of the silica. A lot of the aluminum in our waste is a result of dissolving the fuel pellets, or the target pellets that were aluminum clad but the actual reactor rods were an aluminum-uranium matrix, so aluminum became a byproduct,” he said.
Adding to the toxic mix is iron sulfamate, which was added for reduction reactions, along with chromium, manganese and nickel. Then there’s the salt:
“When you go to carbon steel tanks [with the waste] you can’t add nitric acid to them, so they neutralized the nitric acid with sodium hydroxide. That neutralization step is the source of most of the sodium in the tank waste,” said Stone. “When you add the hydroxide, a lot of the metals will drop out into a metal hydroxide/oxide sludge layer.”
graphic: Alphonzo James, SRNL
When the metals drop out, it leaves a supernate on top and when evaporated, the supernate forms a salt cake, and this is where the salt waste comes from. The supernate is evaporated because it is easier to deal with the salts in its solid form – if left in a liquid state, sites would have to build twice as many storage tanks.
The sludge, salt and supernate would all be classified as high-level waste as stored. According to Stone cesium will be present in the salt waste whereas a lot of the long-lived radionuclides are in the sludge waste.
Today the race is on to process that waste and make its long-term storage less of a threat to the environment, and SRNL is leading that race as the Environmental Management Corporate Laboratory.
Through the years an array of processes including vitrification (incorporating radionuclides in the molecular structure of glass), calcination and sending low level waste into cementitious grout and saltstone were created. The goal is not elimination of the radioactive materials (because it isn’t possible), rather, safely remove liquid waste from tanks and convert the liquid waste into a safer form for long-term storage. This conversion removes the threat to the environment from leaks, spills or leaching and allows for closure of aging waste tanks.
While sites have similar issues with handling liquid waste, they adopted differing processes to deal with it, and those processes have had varying levels of success, cost and efficiency.
“When SRNL started working on the tank waste problem, we were trying to figure out what was the right waste form for the high-level waste,” said Connie Herman, Associate Lab Director for Environmental and Legacy Management at SRNL. “We were looking to solve the DOE problem of what is the right choice, but then we went specific after that and looked at how do we design the equipment, what kind of plant do we need here for our specific tank waste at the Savannah River Site,” she said. “We knew we had to get to glass [vitrification], the question became how to do that here in a remotely operated environment.”
Herman said the technology for putting high-level waste in glass for long term storage was developed in the 1970’s. Construction for the Defense Waste Processing Facility (DWPF) started in 1982, with the facility doing cold operations in 1994 and moving to rad operations in 1996.
“In those 10 years there was some work done in the tank farms to get them ready to send waste for processing,” said Stone. “There were also some aborted techniques in that time span. A sludge washing facility was designed, but not built, because we figured out sludge treatment could be done in the existing tanks,” he said.
“The salt waste or low-level fraction of our waste was going down a process that essentially failed,” said Stone. “The process required salt processing in small batches and initially they tried to do it in one of the tank farm tanks. That led to a lot more benzene than we had anticipated, so for salt processing, we had to rethink our original ideas on how to do that,” said Stone.
Stone said they switched to a solvent extraction process used at the Savannah River Site’s Salt Waste Processing Facility (SWPF). After abandonment of the failed process and before the startup of the SWPF solvent extraction process, implementation of TCCR (Tank Closure Cesium Removal) made substantial changes happen.
“First we had to very quickly develop a way to process at DWPF without the salt waste,” said Stone. “And then we had to incorporate the revised salt processing methods and streams into DWPF.”
Stone said the treatment process to make the low level waste fraction includes a filtration or settling step to ensure none of the sludge solids get into the treated salt waste.
He noted that at SRS they can also treat the soluble actinides by adding monosodium titanate. The monosodium titanate absorbs the actinides and can absorb or dissolve strontium as well. The monosodium titanate is filtered out when the solid step filtration is complete, leaving a relatively clean supernate stream.

The Defense Waste Processing Facility (DWPF) at the Savannah River Site. photo: Savannah River Site Photography
The supernate has a lot of cesium in it at this point, and so a solvent-extraction process (like at SWPF) or CST (crystalline silicotitanate ion exchange) is used to extract the cesium (as was done in TCCR). With the cesium removed from the supernate, the decontaminated salt solution then moves to salt stone as a low-level waste.
Stone said the low-level waste goes into surface disposal or near surface disposal. At SRS that means vaults. Oddly enough, the vaults look a lot like – tanks.
“The original structures were rectangles and looked like vaults, hence the name,” said Stone. “Then they went to ‘coliseums’ – a supersized vault – and it became round but kept the name.”
The vaults are rated for a 500-year life. And while the minute amount of cesium in the salt stone has a very short half-life, species like technetium 99 and iodine 129 are both relatively longlived. The storage system requires the ability to remain below a specific dose release factor for 1,000 years.
While the low-level supernate part of the waste is in saltstone in vaults, the sludge from the tanks, a high-level waste, with all its metals, has a lot of the long-lived radionuclides like plutonium, uranium, and neptunium, requiring a different process.
Stone says the sludge goes through ‘sludge washing’ to remove as much of the soluble materials as practical – the dissolved species are eventually sent to saltstone. Sludge washing reduces the amount of waste going through high-level waste disposal and allows for higher waste loading during vitrification. Washing also helps remove a lot of sulfur, which is problematic in vitrification as it causes a highly corrosive layer due to its limited incorporation in glass.
“So, we go through basically a process to get rid of the highly-concentrated residual supernate and the sludge in the interstitial pockets, as well as to dissolve some of the sulfate and sodium,” said Stone. He also said those batches with high amounts of aluminum can go through a process to dissolve the aluminum and force it into the supernate, which then goes to saltstone as a low-level waste, rather than going to vitrification with the high-level waste.
Stone notes not all sludge batches go through that process. The waste qualification process helps determine how much the sludge should be washed and whether or not aluminum removal is performed. SRNL receives samples from the tank waste, puts it through the washing process and then puts the sample through processing evaluations to evaluate how the sludge will be processed. Qualification of the sludge batches is a requirement for the DWPF to accept a sludge batch.
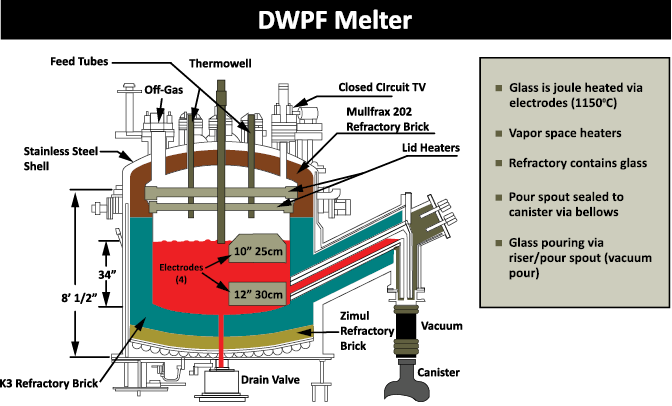
When a sludge batch is accepted at DWPF for vitrification, they will add back any solids that were filtered out at SWPF, as well as the removed cesium and other radionuclides contained in the solvent stream. The sludge is later acidified using a blend of nitric and glycolic acids. It is then boiled to reduce the volume. While at boiling temperature, the nitrite is destroyed, and mercury is reduced to elemental mercury and removed from the waste via steam stripping. Stone said this is where a lot of other redox chemical reactions happen. The process is designed to have a balanced redox, so that about 20 percent of the iron will be in a reduced state in the final glass product. This balanced redox allows for faster glass melting while not being so reducing that metallic species form in the melter.
When boiling is completed, the stream is sent to a tank where it is mixed with frit or pre-determined glass formers that are specific to the batch. The frit is added as a water slurry and then all the water is evaporated off. The boiling leaves the melter feed as concentrated as possible. When it reaches the target solids endpoint a chemical composition evaluation is done. The evaluation shows the chemical composition in this slurry melter feed will make glass with the desired properties.
graphic: Alphonzo James, SRNL
The glass, which more closely resembles obsidian rather than window glass, goes into stainless steel canisters, which are then safely stored for eventual geologic disposal.
While the treatment of tank waste in this article focuses primarily on SRS, it’s important to note that SRNL is working with other DOE sites to implement the lessons learned in tank waste processing to operations at Hanford, Oak Ridge and Idaho.
These processes were developed to clean up the millions of gallons of liquid waste resulting from Cold War production of plutonium and uranium. And while these processes move forward, and waste tanks get closed, SRS and Los Alamos are poised to begin production of plutonium pits to ensure the readiness of the nation’s nuclear arsenal. Fortunately, much has been learned during the past several decades and researchers are already at work to find ways to reduce waste products from weapons production.
“Can we stabilize some of the waste from pit production, in let’s say grout, so we don’t have tanks of liquid waste,” said Herman. Any of the potential secondary waste that might be generated, like plutonium and the transuranic (job control) waste will be evaluated. SRNL is working with the Carlsbad Field Office with the Waste Isolation Pilot Plant to determine if we can dispose of that secondary waste directly to those locations.
“The problem is the original inventory targeted for disposition at the Waste Isolation Pilot Plant was much lower in plutonium, so we are working with the Carlsbad office to evaluate the impact of that additional plutonium,” said Herman. “So yes, we have learned the key part of this is if you design the process with the end in mind and thinking about that you’ve got to generate waste and you’ve got to get rid of the waste and you incorporate the researchers who know how to do that. Then you don’t create as much waste, number one, or in the end you create a waste that’s hopefully got a smaller volume,” she said.
“But that waste stream will be significantly different than the tank waste because you’re not going through spent nuclear fuel reprocessing to get the material that’s going in the pits this time,” said Stone.

DWPF canisters. photo: Savannah River Site photography
Even as SRS and Los Alamos gear up for pit production, and as the National Nuclear Security Administration prepares to take ownership of SRS, SRNL’s environmental management researchers are not only executing DOE-EM’s mission today, but they are also preparing to minimize the risk to our environment with the production of the next generation of plutonium pits.